Kolekce Atom Structure Of Gold
Kolekce Atom Structure Of Gold. Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange).
Nejlepší Gold Atomic Structure Has Atomic Number Stock Vector Royalty Free 1919414927
2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons;79 electrons (white) successively occupy available electron shells (rings).
And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons; These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element.

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons;

And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. These electrons will exist in determined orbitals around the nucleus.. 79), the most common isotope of this element.
79 electrons (white) successively occupy available electron shells (rings).. And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

79), the most common isotope of this element. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element. And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons;

The nucleus consists of 79 protons (red) and 118 neutrons (orange).. . Each orbital can hold a certain amount of electrons;
79 electrons (white) successively occupy available electron shells (rings).. These electrons will exist in determined orbitals around the nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element.. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons;. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

79 electrons (white) successively occupy available electron shells (rings).. Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). 2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. 79 electrons (white) successively occupy available electron shells (rings).

Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus.. 2, 8, 18, 32, 18, 1.

79), the most common isotope of this element. .. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 2, 8, 18, 32, 18, 1. And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element.. 79), the most common isotope of this element.

And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange).

These electrons will exist in determined orbitals around the nucleus. These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1... 2, 8, 18, 32, 18, 1.

79 electrons (white) successively occupy available electron shells (rings)... 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 2, 8, 18, 32, 18, 1. And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold:
The nucleus consists of 79 protons (red) and 118 neutrons (orange)... 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1... Each orbital can hold a certain amount of electrons;

The nucleus consists of 79 protons (red) and 118 neutrons (orange).. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1. These electrons will exist in determined orbitals around the nucleus... 2, 8, 18, 32, 18, 1.

These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange).. And for gold, each energy level will hold:

Each orbital can hold a certain amount of electrons;. 79), the most common isotope of this element. And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus. And for gold, each energy level will hold:

And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus... Each orbital can hold a certain amount of electrons;

Each orbital can hold a certain amount of electrons;.. These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). 79), the most common isotope of this element.

79 electrons (white) successively occupy available electron shells (rings).. These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons;.. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

2, 8, 18, 32, 18, 1. .. These electrons will exist in determined orbitals around the nucleus.
79 electrons (white) successively occupy available electron shells (rings)... 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1.. Each orbital can hold a certain amount of electrons;

79 electrons (white) successively occupy available electron shells (rings).. And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element.. 2, 8, 18, 32, 18, 1.

The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange).. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

2, 8, 18, 32, 18, 1... And for gold, each energy level will hold:

2, 8, 18, 32, 18, 1.. These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold:

2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus... The nucleus consists of 79 protons (red) and 118 neutrons (orange).
79), the most common isotope of this element.. And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings).

These electrons will exist in determined orbitals around the nucleus. These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings).. These electrons will exist in determined orbitals around the nucleus.

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold:

79), the most common isotope of this element. 79), the most common isotope of this element. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons; These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. And for gold, each energy level will hold:

And for gold, each energy level will hold:.. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons;.. And for gold, each energy level will hold:

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1. And for gold, each energy level will hold: And for gold, each energy level will hold:

2, 8, 18, 32, 18, 1. 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus.. 79), the most common isotope of this element.

The nucleus consists of 79 protons (red) and 118 neutrons (orange)... 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold:. 79), the most common isotope of this element.

These electrons will exist in determined orbitals around the nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (orange).

79 electrons (white) successively occupy available electron shells (rings)... 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element. These electrons will exist in determined orbitals around the nucleus.. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (orange).

These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1.. Each orbital can hold a certain amount of electrons;

2, 8, 18, 32, 18, 1... These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

2, 8, 18, 32, 18, 1... And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element.. 79), the most common isotope of this element.

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element. And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons;. These electrons will exist in determined orbitals around the nucleus.

And for gold, each energy level will hold:. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.
2, 8, 18, 32, 18, 1.. 2, 8, 18, 32, 18, 1. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. 2, 8, 18, 32, 18, 1.

And for gold, each energy level will hold:. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons;

The nucleus consists of 79 protons (red) and 118 neutrons (orange). 2, 8, 18, 32, 18, 1. These electrons will exist in determined orbitals around the nucleus.

And for gold, each energy level will hold: . 79 electrons (white) successively occupy available electron shells (rings).

The nucleus consists of 79 protons (red) and 118 neutrons (orange)... Each orbital can hold a certain amount of electrons; These electrons will exist in determined orbitals around the nucleus. And for gold, each energy level will hold:

Each orbital can hold a certain amount of electrons;.. And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings).. 2, 8, 18, 32, 18, 1.

And for gold, each energy level will hold: And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). 79), the most common isotope of this element. Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (orange).

Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings).

Each orbital can hold a certain amount of electrons;.. . These electrons will exist in determined orbitals around the nucleus.

79 electrons (white) successively occupy available electron shells (rings)... These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons;.. These electrons will exist in determined orbitals around the nucleus.

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings).
And for gold, each energy level will hold:.. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. These electrons will exist in determined orbitals around the nucleus. 79 electrons (white) successively occupy available electron shells (rings).

79 electrons (white) successively occupy available electron shells (rings)... These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

79), the most common isotope of this element. Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1.. These electrons will exist in determined orbitals around the nucleus.

And for gold, each energy level will hold:.. And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). 79), the most common isotope of this element... 79 electrons (white) successively occupy available electron shells (rings).

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus... The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus.. 79), the most common isotope of this element.

Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange).. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

The nucleus consists of 79 protons (red) and 118 neutrons (orange).. 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons; These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings).

79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus.. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (orange).

2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Each orbital can hold a certain amount of electrons;

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

Each orbital can hold a certain amount of electrons; Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. These electrons will exist in determined orbitals around the nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). 79), the most common isotope of this element.

2, 8, 18, 32, 18, 1... 79), the most common isotope of this element.

The nucleus consists of 79 protons (red) and 118 neutrons (orange).. And for gold, each energy level will hold: 79), the most common isotope of this element. Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus.

And for gold, each energy level will hold:. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings).

Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. These electrons will exist in determined orbitals around the nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange).. And for gold, each energy level will hold:
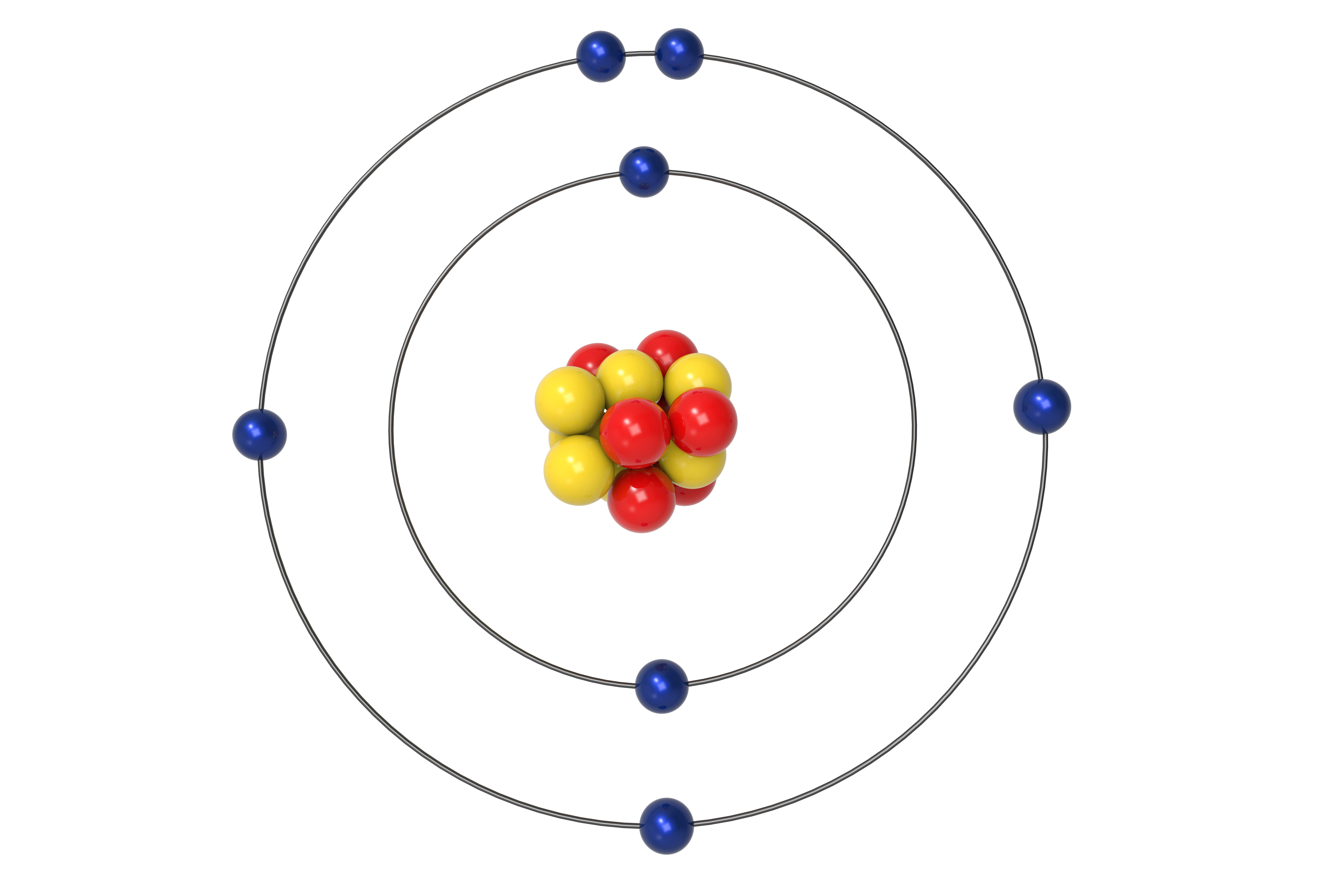
26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. And for gold, each energy level will hold:. 79), the most common isotope of this element.

79), the most common isotope of this element. These electrons will exist in determined orbitals around the nucleus. And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons;. 79 electrons (white) successively occupy available electron shells (rings).

These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. And for gold, each energy level will hold:

The nucleus consists of 79 protons (red) and 118 neutrons (orange).. 2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus... 79), the most common isotope of this element.

79), the most common isotope of this element. 79), the most common isotope of this element.

2, 8, 18, 32, 18, 1.. Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus.. 79), the most common isotope of this element.

2, 8, 18, 32, 18, 1... These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 2, 8, 18, 32, 18, 1... 2, 8, 18, 32, 18, 1.
26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons; 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. And for gold, each energy level will hold:

79), the most common isotope of this element. These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold: 79), the most common isotope of this element. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus.

The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. 2, 8, 18, 32, 18, 1.

These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. And for gold, each energy level will hold:. And for gold, each energy level will hold:

79 electrons (white) successively occupy available electron shells (rings)... 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; And for gold, each energy level will hold:. 79 electrons (white) successively occupy available electron shells (rings).

79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 2, 8, 18, 32, 18, 1. And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons; 79), the most common isotope of this element. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus... The nucleus consists of 79 protons (red) and 118 neutrons (orange).